Benutzer:Jan Lukas Hobrock/FAS
Vorlage:Enzyme Vorlage:Infobox gene Fatty acid synthase (FAS) is an enzyme that in humans is encoded by the FASN gene.[1][2][3][4]
It catalyzes fatty acid synthesis from acetyl-CoA and malonyl-CoA, in the presence of NADPH.[4]. Depending on the species it may exist as set of separate enzymes or an integrated multi-enzyme complex. [5][6][7][8]
Its main function is to catalyze the synthesis of palmitate (C16:0, a long-chain saturated fatty acid)
The fatty acids are synthesized by a series of decarboxylative Claisen condensation reactions from acetyl-CoA and malonyl-CoA. Following each round of elongation the beta keto group is reduced to the fully saturated carbon chain by the sequential action of a ketoreductase (KR), dehydratase (DH), and enoyl reductase (ER). The growing fatty acid chain is carried between these active sites while attached covalently to the phosphopantetheine prosthetic group of an acyl carrier protein (ACP), and is released by the action of a thioesterase (TE) upon reaching a carbon chain length of 16 (palmitic acid).
Classes
There are two principal classes of fatty acid synthases.
- Type I systems utilize a single large, multifunctional complex as observed in animals and fungi (although the structural arrangement of fungal and animal synthases differ). A Type I fatty acid synthase system is also found in the CMN group of bacteria (corynebacteria, mycobacteria, and nocardia). In these bacteria, the FAS I system produces palmitic acid, and cooperates with the FAS II system to produce a greater diversity of lipid products.[9]
- Type II is found in plants, archaea and bacteria (including plastids and mitochondria), and is characterized by the use of discrete, monofunctional enzymes for fatty acid synthesis. Inhibitors of this pathway (FAS II) are being investigated as possible antibiotics.[10]
The mechanism of FAS I and FAS II elongation and reduction is the same, as the domains of the FAS II enzymes are largely homologous to their domain counterparts in FAS I multienzyme polypeptides. However, the differences in the organization of the enzymes - integrated in FAS I, discrete in FAS II - gives rise to many important biochemical differences.[11]
The evolutionary history of fatty acid synthases are very much intertwined with that of polyketide synthases (PKS). Polyketide synthases use a similar mechanism and homologous domains to produce secondary metabolite lipids. Furthermore, polyketide synthases also exhibit a Type I and Type II organization. FAS I in animals is thought to have arisen through modification of PKS I in fungi, whereas FAS I in fungi and the CMN group of bacteria seem to have arisen separately through the fusion of FAS II genes.[9]
Structure and mechanism of action
For a more detailed discussion of the mechanism see Fatty acid synthesis#Straight-chain fatty acids.
Mammalian FAS
Mammalian FAS consists of a homodimer, in which three catalytic domains in the N-terminal section (-ketoacyl synthase (KS), malonyl/acetyltransferase (MAT), and dehydratase (DH)), are separated by a core region of 600 residues from four C-terminal domains (enoyl reductase (ER), -ketoacyl reductase (KR), acyl carrier protein (ACP) and thioesterase (TE)).[12][13] Synthesis of fatty acids is prepared by transfer of an acetyl group from Acetyl-CoA first to the phosphopantetheine prosthetic group of the acyl carrier protein and from there on ketoacyl synthase (KS) by malonyl/acetyltransferase. The same domain catalyzes the analogous transfer of a malonyl group from Malonyl-CoA to the unoccupied acyl carrier protein (ACP). The first reaction of the reaction cycle, elongation, is a nucleophilic attack of the carbon adjacent to the acid group in malonyl-ACP on the thioester of acetyl-KS yielding beta-ketoacyl-ACP which is catalyzed by KS. This reaction is energetically unfavorable but driven forward by release of CO2 explaining the need for Malonyl-CoA (as opposed to another molecule of Acetyl-CoA) as the nucleophile. Next, beta-ketoacyl-ACP is reduced to beta-D-hydroxyacyl-ACP by beta-ketoacyl-ACP consuming one equivalent of NADPH and a proton. Subsequent elimination of a molecule of water, catalysed by beta D hydroxyacyl-ACP dehydratase yields trans DELTA2 Acyl-ACP, which is NADPH/H+-dependently reduced by action of enoyl-ACP reductase to yield the fully saturated carboxylic acid bond to ACP as a thioester. The aforementioned steps are reiterated until the thioester bond is either hydrolysed by thioesterase to release the fatty acid (most organisms) or broken and reformed with CoA by a malonyl/acyl-transferase producing fatty acid-CoA (fungi).
History
The conventional model for organization of FAS (see the 'head-to-tail' model on the right) is largely based on the observations that the bifunctional reagent 1,3-dibromopropanone (DBP) is able to crosslink the active site cysteine thiol of the KS domain in one FAS monomer with the phosphopantetheine prosthetic group of the ACP domain in the other monomer.[14][15] Complementation analysis of FAS dimers carrying different mutations on each monomer has established that the KS and MAT domains can cooperate with the ACP of either monomer.[16][17] and a reinvestigation of the DBP crosslinking experiments revealed that the KS active site Cys161 thiol could be crosslinked to the ACP 4'-phosphopantetheine thiol of either monomer.[18] In addition, it has been recently reported that a heterodimeric FAS containing only one competent monomer is capable of palmitate synthesis.[19]
The above observations seemed incompatible with the classical 'head-to-tail' model for FAS organization, and an alternative model has been proposed, predicting that the KS and MAT domains of both monomers lie closer to the center of the FAS dimer, where they can access the ACP of either subunit (see figure on the top right).[20]
A low resolution X-ray crystallography structure of both pig (homodimer)[21] and yeast FAS (heterododecamer)[22] along with a ~6 Å resolution electron cryo-microscopy (cryo-EM) yeast FAS structure [23] have been solved.
Substrate shuttling mechanism
The solved structures of yeast FAS and mammalian FAS show two distinct organizations of highly conserved catalytic domains/enzymes in this multi-enzyme cellular machine. Yeast FAS has a highly efficient rigid barrel-like structure with 6 reaction chambers which synthesize fatty acids independently, while the mammalian FAS has an open flexible structure with only two reaction chambers. However, in both cases the conserved ACP acts as the mobile domain responsible for shuttling the intermediate fatty acid substrates to various catalytic sites. A first direct structural insight into this substrate shuttling mechanism was obtained by cryo-EM analysis, where ACP is observed bound to the various catalytic domains in the barrel-shaped yeast fatty acid synthase.[23] The cryo-EM results suggest that the binding of ACP to various sites is asymmetric and stochastic, as also indicated by computer-simulation studies[24]
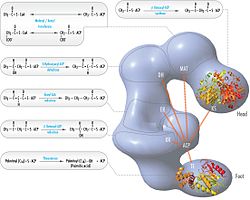 FAS revised model with positions of polypeptides, three catalytic domains and their corresponding reactions, visualization by Kosi Gramatikoff. Note that FAS is only active as a homodimer rather than the monomer pictured. |
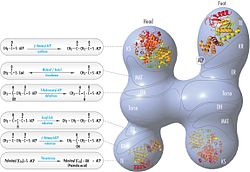 FAS 'head-to-tail' model with positions of polypeptides, three catalytic domains and their corresponding reactions, visualization by Kosi Gramatikoff. |
Regulation
Metabolism and homeostasis of fatty acid synthase is transcriptionally regulated by Upstream Stimulatory Factors (USF1 and USF2) and sterol regulatory element binding protein-1c (SREBP-1c) in response to feeding/insulin in living animals.[25][26]
Although liver X receptor (LXRs) modulate the expression of sterol regulatory element binding protein-1c (SREBP-1c) in feeding, regulation of FAS by SREBP-1c is USF-dependent.[26][27][28][29]
Acylphloroglucinols isolated from the fern Dryopteris crassirhizoma show a fatty acid synthase inhibitory activity.[30]
Clinical significance
The gene that codes for FAS has been investigated as a possible oncogene.[31] FAS is upregulated in breast and gastric cancers, as well as being an indicator of poor prognosis may also be worthwhile as a chemotherapeutic target.[32][33][34] FAS inhibitors are therefore an active area of drug discovery research.[35][36][37][38]
FAS may also be involved in the production of an endogenous ligand for the nuclear receptor PPARalpha, the target of the fibrate drugs for hyperlipidemia,[39] and is being investigated as a possible drug target for treating the metabolic syndrome.[40] Orlistat which is a gastrointestinal lipase inhibitor also inhibits FAS and has a potential as a medicine for cancer.[41][42]
In some cancer cell lines, this protein has been found to be fused with estrogen receptor alpha (ER-alpha), in which the N-terminus of FAS is fused in-frame with the C-terminus of ER-alpha.[4]
An association with uterine leiomyomata has been reported.[43]
See also
- Discovery and development of gastrointestinal lipase inhibitors
- Fatty acid synthesis
- Fatty acid metabolism
- Fatty acid degradation
- Enoyl-acyl carrier protein reductase
- List of fatty acid metabolism disorders
References
Further reading
- Wakil SJ: Fatty acid synthase, a proficient multifunctional enzyme. In: Biochemistry. 28, Nr. 11, 1989, S. 4523–4530. doi:10.1021/bi00437a001. PMID 2669958.
- Fatty acid synthase: a metabolic oncogene in prostate cancer?. In: Journal of Cellular Biochemistry. 91, Nr. 1, 2004, S. 47–53. doi:10.1002/jcb.10708. PMID 14689581.
- Lejin D: [Viscosimetry in clinical practice]. In: Medicinski Pregled. 30, Nr. 9–10, 1978, S. 477–482. PMID 600212.
- Wronkowski Z: [Cancer diagnosis of the respiratory system]. In: Pielȩgniarka I Połozna. Nr. 12, 1976, S. 7–8. PMID 1044453.
- Human fatty acid synthase mRNA: tissue distribution, genetic mapping, and kinetics of decay after glucose deprivation. In: Journal of Lipid Research. 36, Nr. 7, 1995, S. 1507–1521. PMID 7595075.
- Fatty acid synthesis: a potential selective target for antineoplastic therapy. In: Proceedings of the National Academy of Sciences of the United States of America. 91, Nr. 14, 1994, S. 6379–6383. bibcode:1994PNAS...91.6379K. doi:10.1073/pnas.91.14.6379. PMID 8022791. PMC 44205 (freier Volltext).
- Human fatty-acid synthase gene. Evidence for the presence of two promoters and their functional interaction. In: Journal of Biological Chemistry. 271, Nr. 23, 1996, S. 13584–13592. doi:10.1074/jbc.271.23.13584. PMID 8662758.
- Expression of fatty acid synthase is closely linked to proliferation and stromal decidualization in cycling endometrium. In: International Journal of Gynecological Pathology. 16, Nr. 1, 1997, S. 45–51. doi:10.1097/00004347-199701000-00008. PMID 8986532.
- Human fatty acid synthase: assembling recombinant halves of the fatty acid synthase subunit protein reconstitutes enzyme activity. In: Proceedings of the National Academy of Sciences of the United States of America. 94, Nr. 23, 1997, S. 12326–12330. bibcode:1997PNAS...9412326J. doi:10.1073/pnas.94.23.12326. PMID 9356448. PMC 24928 (freier Volltext).
- Fatty acid synthase is expressed mainly in adult hormone-sensitive cells or cells with high lipid metabolism and in proliferating fetal cells. In: Journal of Histochemistry and Cytochemistry. 48, Nr. 5, 2000, S. 613–622. doi:10.1177/002215540004800505. PMID 10769045.
- Identification of a novel FAS/ER-alpha fusion transcript expressed in human cancer cells. In: Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression. 1493, Nr. 3, 2000, S. 373–377. doi:10.1016/s0167-4781(00)00202-5. PMID 11018265.
- FIST/HIPK3: a Fas/FADD-interacting serine/threonine kinase that induces FADD phosphorylation and inhibits fas-mediated Jun NH(2)-terminal kinase activation. In: Journal of Experimental Medicine. 192, Nr. 8, 2000, S. 1165–1174. doi:10.1084/jem.192.8.1165. PMID 11034606. PMC 2311455 (freier Volltext).
- Human fatty acid synthase: role of interdomain in the formation of catalytically active synthase dimer. In: Proceedings of the National Academy of Sciences of the United States of America. 98, Nr. 6, 2001, S. 3104–3108. bibcode:2001PNAS...98.3104C. doi:10.1073/pnas.051635998. PMID 11248039. PMC 30614 (freier Volltext).
- Quaternary structure of human fatty acid synthase by electron cryomicroscopy. In: Proceedings of the National Academy of Sciences of the United States of America. 99, Nr. 1, 2002, S. 138–143. bibcode:2002PNAS...99..138B. doi:10.1073/pnas.012589499. PMID 11756679. PMC 117528 (freier Volltext).
- Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. In: Journal of Biological Chemistry. 277, Nr. 13, 2002, S. 11019–11025. doi:10.1074/jbc.M111041200. PMID 11790787.
- Domain movements in human fatty acid synthase by quantized elastic deformational model. In: Proceedings of the National Academy of Sciences of the United States of America. 99, Nr. 12, 2002, S. 7895–7899. bibcode:2002PNAS...99.7895M. doi:10.1073/pnas.112222299. PMID 12060737. PMC 122991 (freier Volltext).
- Polyunsaturated fatty acids decrease the expression of sterol regulatory element-binding protein-1 in CaCo-2 cells: effect on fatty acid synthesis and triacylglycerol transport. In: Biochemical Journal. 368, Nr. Pt 3, 2003, S. 855–864. doi:10.1042/BJ20020731. PMID 12213084. PMC 1223029 (freier Volltext).
External links
- MeSH Jan Lukas Hobrock/FAS
- Fatty Acid Synthesis: Rensselaer Polytechnic Institute
- Fatty Acid Synthase: RCSB PDB Molecule of the Month
- 3D electron microscopy structures of fatty acid synthase from the EM Data Bank(EMDB)
- PDBe-KB provides an overview of all the structure information available in the PDB for Human Fatty acid synthase
Vorlage:PDB Gallery Vorlage:Multienzyme complexes Vorlage:Lipid metabolism enzymes Vorlage:Acyltransferases Vorlage:Enzymes Vorlage:Portal bar
Category:Transferases Category:EC 2.3.1 Category:Metabolism Category:Fatty acids Category:NADPH-dependent enzymes Category:Enzymes of known structure
- ↑ Isolation and chromosomal mapping of genomic clones encoding the human fatty acid synthase gene. In: Genomics. 23, Nr. 2, February 1995, S. 420–424. doi:10.1006/geno.1994.1518. PMID 7835891.
- ↑ Human fatty acid synthase: properties and molecular cloning. In: Proceedings of the National Academy of Sciences of the United States of America. 92, Nr. 19, Oct 1995, S. 8695–8699. bibcode:1995PNAS...92.8695J. doi:10.1073/pnas.92.19.8695. PMID 7567999. PMC 41033 (freier Volltext).
- ↑ The SDR (short-chain dehydrogenase/reductase and related enzymes) nomenclature initiative. In: Chemico-Biological Interactions. 178, Nr. 1–3, Feb 2009, S. 94–98. doi:10.1016/j.cbi.2008.10.040. PMID 19027726. PMC 2896744 (freier Volltext).
- ↑ a b c Entrez Gene: FASN fatty acid synthase.
- ↑ Regulation of synthesis of hepatic fatty acid synthetase: binding of fatty acid synthetase antibodies to polysomes. In: Proceedings of the National Academy of Sciences of the United States of America. 72, Nr. 10, October 1975, S. 3956–3960. bibcode:1975PNAS...72.3956A. doi:10.1073/pnas.72.10.3956. PMID 1060077. PMC 433116 (freier Volltext).
- ↑ Presence of two polypeptide chains comprising fatty acid synthetase. In: Proceedings of the National Academy of Sciences of the United States of America. 72, Nr. 5, May 1975, S. 1940–1944. bibcode:1975PNAS...72.1940S. doi:10.1073/pnas.72.5.1940. PMID 1098047. PMC 432664 (freier Volltext).
- ↑ Specific release of the thioesterase component of the fatty acid synthetase multienzyme complex by limited trypsinization. In: Proceedings of the National Academy of Sciences of the United States of America. 73, Nr. 4, April 1976, S. 1184–1188. bibcode:1976PNAS...73.1184S. doi:10.1073/pnas.73.4.1184. PMID 1063400. PMC 430225 (freier Volltext).
- ↑ Structural and functional organization of the animal fatty acid synthase. In: Progress in Lipid Research. 42, Nr. 4, July 2003, S. 289–317. doi:10.1016/S0163-7827(02)00067-X. PMID 12689621.
- ↑ a b Evolutionary implications of bacterial polyketide synthases. In: Molecular Biology and Evolution. 22, Nr. 10, October 2005, S. 2027–2039. doi:10.1093/molbev/msi193. PMID 15958783.
- ↑ Fulmer T: Not so FAS. In: Science-Business EXchange. 2, Nr. 11, March 2009, S. 430. doi:10.1038/scibx.2009.430.
- ↑ Fundamentals of enzymology: the cell and molecular biology of catalytic proteins. Oxford University Press, Oxford [Oxfordshire] 1999, ISBN 978-0-19-850229-6.
- ↑ Human fatty acid synthase: role of interdomain in the formation of catalytically active synthase dimer. In: Proceedings of the National Academy of Sciences of the United States of America. 98, Nr. 6, March 2001, S. 3104–3108. bibcode:2001PNAS...98.3104C. doi:10.1073/pnas.051635998. PMID 11248039. PMC 30614 (freier Volltext).
- ↑ Smith S: The animal fatty acid synthase: one gene, one polypeptide, seven enzymes. In: FASEB Journal. 8, Nr. 15, December 1994, S. 1248–1259. doi:10.1096/fasebj.8.15.8001737. PMID 8001737.
- ↑ Animal fatty acid synthetase. A novel arrangement of the beta-ketoacyl synthetase sites comprising domains of the two subunits. In: Journal of Biological Chemistry. 256, Nr. 10, May 1981, S. 5128–5133. PMID 6112225.
- ↑ Animal fatty acid synthetase. Identification of the residues comprising the novel arrangement of the beta-ketoacyl synthetase site and their role in its cold inactivation. In: Journal of Biological Chemistry. 257, Nr. 6, March 1982, S. 3230–3235. PMID 7061475.
- ↑ Differential affinity labeling of the two subunits of the homodimeric animal fatty acid synthase allows isolation of heterodimers consisting of subunits that have been independently modified. In: Journal of Biological Chemistry. 273, Nr. 9, February 1998, S. 4937–4943. doi:10.1074/jbc.273.9.4937. PMID 9478938.
- ↑ Mapping the functional topology of the animal fatty acid synthase by mutant complementation in vitro. In: Biochemistry. 40, Nr. 36, September 2001, S. 10792–18799. doi:10.1021/bi015535z. PMID 11535054.
- ↑ Dibromopropanone cross-linking of the phosphopantetheine and active-site cysteine thiols of the animal fatty acid synthase can occur both inter- and intrasubunit. Reevaluation of the side-by-side, antiparallel subunit model. In: Journal of Biological Chemistry. 274, Nr. 17, April 1999, S. 11557–11563. doi:10.1074/jbc.274.17.11557. PMID 10206962.
- ↑ Engineering of an active animal fatty acid synthase dimer with only one competent subunit. In: Chemistry and Biology. 10, Nr. 2, February 2003, S. 169–173. doi:10.1016/S1074-5521(03)00023-1. PMID 12618189.
- ↑ Structure and molecular organization of mammalian fatty acid synthase. In: Nature Structural and Molecular Biology. 12, Nr. 3, March 2005, S. 225–232. doi:10.1038/nsmb899. PMID 15711565.
- ↑ The crystal structure of a mammalian fatty acid synthase. In: Science. 321, Nr. 5894, September 2008, S. 1315–1322. bibcode:2008Sci...321.1315M. doi:10.1126/science.1161269. PMID 18772430.
- ↑ The crystal structure of yeast fatty acid synthase, a cellular machine with eight active sites working together. In: Cell. 129, Nr. 2, April 2007, S. 319–332. doi:10.1016/j.cell.2007.03.013. PMID 17448991.
- ↑ a b Direct structural insight into the substrate-shuttling mechanism of yeast fatty acid synthase by electron cryomicroscopy. In: Proceedings of the National Academy of Sciences of the United States of America. 107, Nr. 20, May 2010, S. 9164–9169. bibcode:2010PNAS..107.9164G. doi:10.1073/pnas.0913547107. PMID 20231485. PMC 2889056 (freier Volltext).
- ↑ Mechanism of substrate shuttling by the acyl-carrier protein within the fatty acid mega-synthase. In: Journal of the American Chemical Society. 132, Nr. 35, September 2010, S. 12357–12364. doi:10.1021/ja103354w. PMID 20704262.
- ↑ Hormonal regulation of mouse fatty acid synthase gene transcription in liver. In: Journal of Biological Chemistry. 264, Nr. 1, January 1989, S. 574–577. PMID 2535847.
- ↑ a b Occupancy and function of the -150 sterol regulatory element and -65 E-box in nutritional regulation of the fatty acid synthase gene in living animals. In: Molecular and Cellular Biology. 23, Nr. 16, August 2003, S. 5896–5907. doi:10.1128/MCB.23.16.5896-5907.2003. PMID 12897158. PMC 166350 (freier Volltext).
- ↑ Direct interaction between USF and SREBP-1c mediates synergistic activation of the fatty-acid synthase promoter. In: Journal of Biological Chemistry. 282, Nr. 8, February 2007, S. 5453–5467. doi:10.1074/jbc.M610566200. PMID 17197698.
- ↑ Identification of liver X receptor-retinoid X receptor as an activator of the sterol regulatory element-binding protein 1c gene promoter. In: Molecular and Cellular Biology. 21, Nr. 9, May 2001, S. 2991–3000. doi:10.1128/MCB.21.9.2991-3000.2001. PMID 11287605. PMC 86928 (freier Volltext).
- ↑ Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. In: Genes and Development. 14, Nr. 22, November 2000, S. 2819–2830. doi:10.1101/gad.844900. PMID 11090130. PMC 317055 (freier Volltext).
- ↑ Fatty acid synthase inhibitory activity of acylphloroglucinols isolated from Dryopteris crassirhizoma. In: Bioorganic & Medicinal Chemistry Letters. 16, Nr. 18, September 2006, S. 4738–4742. doi:10.1016/j.bmcl.2006.07.018. PMID 16870425.
- ↑ Fatty acid synthase: a metabolic oncogene in prostate cancer?. In: Journal of Cellular Biochemistry. 91, Nr. 1, January 2004, S. 47–53. doi:10.1002/jcb.10708. PMID 14689581.
- ↑ MRNA stability and overexpression of fatty acid synthase in human breast cancer cell lines. In: Anticancer Research. 27, Nr. 1A, 2007, S. 27–34. PMID 17352212.
- ↑ Increased expression of fatty acid synthase (OA-519) in ovarian neoplasms predicts shorter survival. In: Human Pathology. 28, Nr. 6, June 1997, S. 686–692. doi:10.1016/S0046-8177(97)90177-5. PMID 9191002.
- ↑ Clinical importance of FASN in relation to HIF-1α and SREBP-1c in gastric adenocarcinoma. In: Life Sciences. 224, May 2019, S. 169–176. doi:10.1016/j.lfs.2019.03.056. PMID 30914315.
- ↑ First Human Study Taking Place With Fatty Acid Synthase Inhibitor. oncotherapynetwork.com. April 7, 2017.
- ↑ Design and synthesis of a series of bioavailable fatty acid synthase (FASN) KR domain inhibitors for cancer therapy. In: Bioorganic & Medicinal Chemistry Letters. 28, Nr. 12, May 2018, S. 2159–2164. doi:10.1016/j.bmcl.2018.05.014. PMID 29779975.
- ↑ A human fatty acid synthase inhibitor binds β-ketoacyl reductase in the keto-substrate site. In: Nature Chemical Biology. 10, Nr. 9, September 2014, S. 774–779. doi:10.1038/nchembio.1603. PMID 25086508.
- ↑ Understanding the Intersections between Metabolism and Cancer Biology. In: Cell. 168, Nr. 4, February 2017, S. 657–669. doi:10.1016/j.cell.2016.12.039. PMID 28187287. PMC 5329766 (freier Volltext).
- ↑ Identification of a physiologically relevant endogenous ligand for PPARalpha in liver.. In: Cell. 138, Nr. 3, August 2009, S. 476–488. doi:10.1016/j.cell.2009.05.036. PMID 19646743. PMC 2725194 (freier Volltext).
- ↑ Antidiabetic and antisteatotic effects of the selective fatty acid synthase (FAS) inhibitor platensimycin in mouse models of diabetes.. In: Proceedings of the National Academy of Sciences of the United States of America. 108, Nr. 13, March 2011, S. 5378–5383. bibcode:2011PNAS..108.5378W. doi:10.1073/pnas.1002588108. PMID 21389266. PMC 3069196 (freier Volltext).
- ↑ Fatty acid synthase as a potential therapeutic target in cancer. In: Future Oncology. 6, Nr. 4, April 2010, S. 551–562. doi:10.2217/fon.10.11. PMID 20373869. PMC 3197858 (freier Volltext).
- ↑ Synthesis of novel beta-lactone inhibitors of fatty acid synthase. In: Journal of Medicinal Chemistry. 51, Nr. 17, September 2008, S. 5285–5296. doi:10.1021/jm800321h. PMID 18710210. PMC 3172131 (freier Volltext).
- ↑ Genome-wide linkage and association analyses implicate FASN in predisposition to uterine leiomyomata. In: American Journal of Human Genetics. 91, Nr. 4, 2012, S. 621–628. doi:10.1016/j.ajhg.2012.08.009. PMID 23040493. PMC 3484658 (freier Volltext).